
Delta enhances the case assembly method for MEA-250A24C H-A from “glue” to “screw and ultrasonic” a solid structure for medical power supply adapter. The safety certificates are updated to reflect this change.
Product change details is as below and the implementation date is 2021/01/01.
| Physical Dimension Change | |
| BEFORE | AFTER |
 | 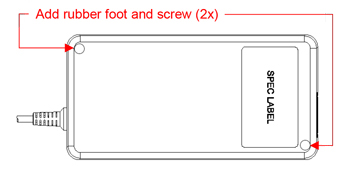 |
| Spec Label Revision Change | |
| BEFORE (Rev. 02) | AFTER (Rev. 03) |
 |  |








